SNELSON REPRESENTS
ELECTRON PAIRING
IN TWO WAYS
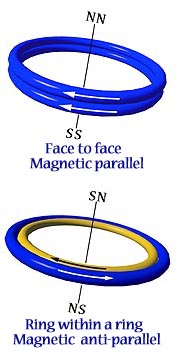
In addition to lying edge to edge in antiparallel, there are two alternate ways for ring-shaped magnets to form attractive relationships.
1. If two magnets are placed one on top of another, in parallel, they will attract face to face. (Fig. on left) The fields add together as a double strength magnet.
2. If two magnets are of different diameters, so that one can fit within the other as a ring within a ring, they will attract if antiparallel. (Fig. on right) If the magnets are of the same strength, they cancel one another to zero.
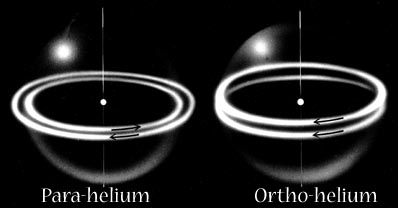
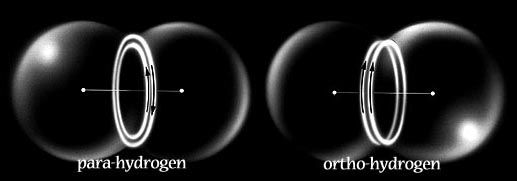
In Snelson's model these become the two modes of magnetic attraction by which electrons can pair together, either in the covalent bond or in the outer shell configurations of the noble gases.