Portrait of an
Atom
(from an exhibition booklet)
by Kenneth Snelson
BACKGROUND AND INTENT
This portrait comes from a tradition. Artists have often shown us the invisible; gods, spirits, goblins and demons. They have made tableaux of epic stories or battle scenes whose witnesses have long disappeared.
The details of the atom's structure are equally invisible and must be conjectured from scientific information. People have sought meaningful images of it since the Greek philosophers first conceived of atomism twenty-five hundred years ago.
Because it is my work to imagine and build sculptures from physical forces, the electronic atom's form and workings have seemed a kind of sculptural riddle, and as I see it, one not yet solved convincingly by science.

In the first quarter of this century there was great hope for achieving the goal of picturing the building block of matter. There were numerous models, some successful others not. There was J.J. Thomson's "raisin in pudding" model and G.N. Lewis's cubical octet atom as well as Parson's magneton electron atom. But great expectations began to blossom with the successes of the Rutherford-Bohr planetary model of 1913. It went through revisions in the decade which followed.
Finally, with Louis de Broglie's 1924 theory of the matter-wave electron, though no one could have anticipated it, the scientific world, by a surprising turn, was about to lay to rest its long search for the "real" and pictorial model which people had thought about for over two dozen centuries.
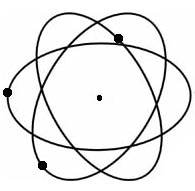
This reversal is generally attributed to the German physicist Werner Heisenberg's important discovery in 1926 that it is impossible to determine in one and the same experiment both the momentum and the position of an electron. Since to "follow an electron in its orbit" had been the physicist's aim, and because this now was seen as an impossibility, the entire proposition needed rethinking. What was an atomic model? It was now reasoned that for scientists to speculate on atomic problems beyond the realm which could be verified was not truly scientific.
By 1930 the uncertainty principle and its implications became a hot issue. It led to much debate and even to some name-calling. It came to be that anyone who argued against the new order was thought of either as naive or as an old fogy -- a person from the previous century. Any model which delved into questions of how electrons might actually move within the atom was seen as heretical.
The classical search for cause and effect was replaced by a view that the atom was an acausal device. Its intricacies, what ever they might be, could be discussed only statistically, as millions of tiny micro-accidents and as the mathematical probability of hypothetically locating an electron within the atom -- if there were some means to look for it.
A few well known physicists held hope for a better model in the future. "I do not believe that God throws dice," was Einstein's often quoted declaration.
Irwin Schroedinger, whose famous wave equation is the cornerstone of the statistical view, strongly objected to the interpretation given his work. Commenting on the uncertainty principle he said, "It seemed to relieve us from the search for what I should call real understanding. It even rendered the endeavor suspect, as betraying an unphilosophical mind -- the mind of a child who regretted the loss of its favorite toy (the picture or model) and would not realize that it was gone forever."
Max Born, one of the founders and staunch advocates of the new orthodoxy, described it in this way: "What lies within the limits (of the uncertainty principle) is knowable. It is a world of experience, wide, rich enough in changing hues and patterns to allure us to explore it in all directions. What lies beyond, the dry tracts of metaphysics, we willingly leave to speculative philosophy."
These dry tracts have lain unclaimed for five decades. From my view it may be that artists are the last of the speculative philosophers -- at least the kind Max Born was speaking of. Even though this territory is rejected by science, it remains the place we might one day find out what an atom would be like in a photographic facsimile or a sculptured replica just as we have learned about the DNA molecule, for example.
My point of departure for a visualized atom is precisely where de Broglie's picture left off. His was the last of the "physical" models. I have taken his matter-wave electron as a physical fact. What I introduce is the hypothesis that the electron's matter-wave orbit has that special property of gross matter by which it occupies its own exclusive space. This means that the entire pathway of the electron can push and otherwise limit the space of the other electrons within the atom much as whole atoms do in relation to one another. Starting with this assumption the atom begins to make sense as an electromagnetic and mechanical object.
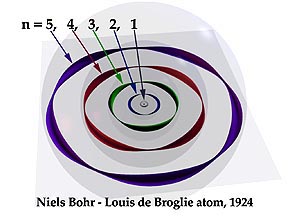
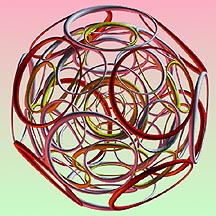
The features of my picture come close to those we should expect of an atom in order to see it as a workable device capable of doing all those remarkable things an atom is able to do. It must give off and receive light like a tiny space station. It can remain stable and resist collapse under great pressure. It collects and organizes its electrons in shells around the nucleus. It puts to use all of its electrical, dynamic and magnetic forces in it structure. It can attach itself to other atoms in molecules and crystals with astonishing virtuosity. And though its electrons are in rapid and perpetual motion, it can sit in tranquility in a rock for eternity.
My new model presents a structure which would do all these things. If it turns out to have nothing to do with real atoms then it is simply an incredible invention of the mind. Its unfamiliar appearance may be irritating to those accustomed to the usual images. "This is not what the way an atom ought to look!" said one famous physicist. However it is viewed I hope people will find it interesting and most of all thought-provoking.
I wish to thank the National Endowment for the Arts for its support in making this exhibition possible.
Kenneth Snelson,
November 24, 1980