|
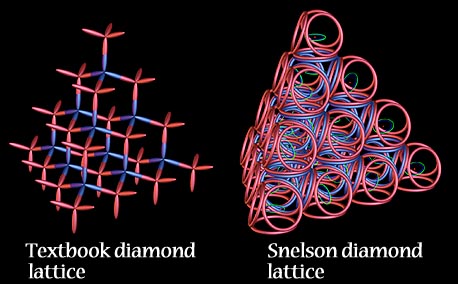
THE DIAMOND is a giant molecule made only of carbon atoms bound together by covalent bonds, in the arrangement shown at left.
The strength of the diamond, in Snelson’s model, is attributed to the matter-wave orbits’ impenetrability, along with the covalent bonds. “The paradox of the diamond is interesting. Its atoms are not arranged in a tight, closest-packing order. They lack the triangulation of sound architecture. In order for its remarkable rigidity to be understood, I assume that the electrons which surround this meager structure supply it with its resistance to deformation. This is one reason why I cannot assume that electron clouds can infiltrate one another like vapors or ghosts.” |
|
COMPLEX ATOMS WITH MANY ELECTRONS |
|
| Complex atoms are arranged as concentric spheres. Proper numbers of electrons in shells are included according to the requirements of the periodic table of elements.
Subshells are provisional arrangements and can combine with other subshells to form more spatially economical structures. For example, beyond the first shell, the two “s” electrons and the six “p” electrons are integrated into an eight-magnet octahedral form in Snelson’s model. Because of the intense nuclear attraction in the inner shells of heavy atoms, all electrons are forced to occupy one-wave orbits which carry the maximum of orbital magnetism. They form a linking group around the sphere with whichever magnetic mosaic is most economical. As in a game of musical chairs, those unable to enter try to find a place in the next higher shell. Near the surface of the atom, where the nuclear attraction is less intense, electrons provide one another more freedom to change their relationships by using orbits of more than one wave. It is in the outermost shell that chemical bonding can occur. |
 |